We are excited to start a new educational series on explaining our approaches in biomedical research, which we hope will be useful to the ME/CFS and chronic illness community; both to patients as well as physicians.
For our first topic, we have chosen to explain Flow Cytometry and FACS (Fluorescence-Activated Cell Sorting). This will be a mini-series of several blog articles, and is not intended to be too detailed or to serve as an academic introduction, as there are many videos and introductions online that serve that purpose. Instead, we hope to provide a sense of what Flow Cytometry is and how we use it in our research at the JAX CRC.
So why start with Flow Cytometry? For immunologists like us, this instrument is indispensable because we deal with complex mixtures of cells from blood or tissues, and flow cytometry allows us to determine the composition, frequency, and function of each of these different cell types. By identifying specific cell types, we are then able to sort these cell types into pure populations that we can use in downstream experiments or interrogations. Indeed, this technology can sort out any type of cell (even including bacterial cells), provided there is a probe you can use to specifically identify them.
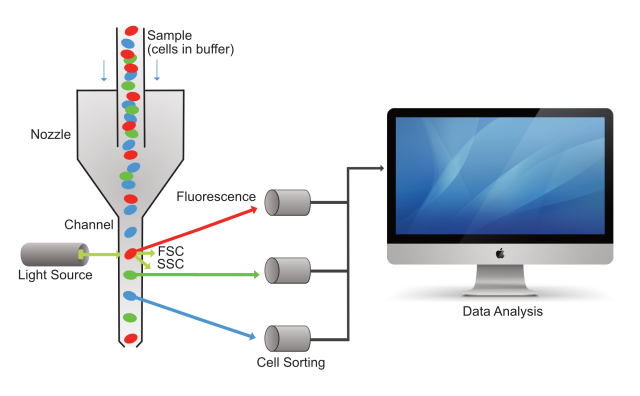
Figure 1. Pictorial of a cell sample moving through the flow cytometer, with cells being sorted based on the fluorescence color they emit, and all the data captured by a computer. FSC is Forward Scattered Light, and SSC is Side Scattered Light.
How does Flow Cytometry work?
In the simplest terms, Flow Cytometry uses laser light to detect the shape, granularity, and fluorescence from a cell. It is a very powerful method because it can do this on thousands of cells per second and accurately sort them out using many parameters simultaneously.
So how does it really work? Cells are first suspended in a fluid (we call it a buffer), and then passed through a narrow channel one at a time at the tremendous speed of thousands of cells per second. A laser light is directed into this channel, and as it hits the cells while they are passing through, it deflects off of the cells, and also emits fluorescence based on molecules present in or on the cells. A series of sensors and filters are then used to sort the cells based on the color of their fluorescence, as well as to turn this information into electronic data (see figure 1, above). The data can then be visualized and further analyzed using a specific software which we will explain in more detail in the next article in this series.
Collecting this data using the flow cytometer is called “acquisition,” and is done by a computer and dedicated software, which also handles the digital interface with the cytometer. The fluorescence collected is typically referred to as “colors,” since each fluorescent molecule emits a different color in the light spectrum.
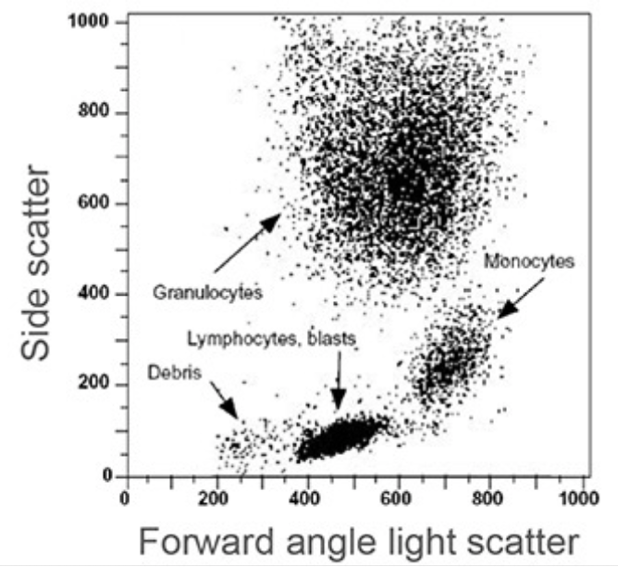
Figure 2. Example data output by the flow cytometry software. Each dot represents an individual cell analyzed by the flow cytometer. This will be explained more in-depth in the next article in the series. Image Reference: Riley and Idowu, Principles and Applications of Flow Cytometry.
What are some different types of flow cytometry instruments?
There are now different types of flow cytometers, some capable of sorting out 20-30 “colors” at a time, and others capable of sorting samples or performing high-throughput analysis. Here at the Jackson Laboratory, we are very fortunate to have some of the best state-of-the-art flow cytometry machines available. Below are photos of some of the instruments we use on a daily basis at our core facilities.
The FACSymphony A5 has 5 lasers, and up to 28 colors that can be used during a single experiment. This system allows researchers to precisely identify and characterize cell populations.

Fortessa X-20 has 4 lasers, with up to 16 colors. Less capable than FACSSymphony, this system is an affordable choice with a compact footprint.

The Sony SP6800 Spectral Analyzer is a 3 laser, 20 color spectral cytometer. It collects a wide spectrum of fluorescence. It has many advantages in analyzing data.

The iQue Screener Plus Intellicyt analyzes up to 13 colors in high-throughput screens through 96- or 384-well sample plates using robotics, capable of running thousands of samples in a single experiment.

Stay tuned for the second part of this series, where we will be discussing the type of data acquired by Flow Cytometry, and how we interpret it from an immunological stand-point. We also welcome suggestions about this article and for new topics.
References:
Introduction to Flow Cytometry, Abcam
Flow Cytometry Data Analysis, Biorad
What is Flow Cytometry (FACS Analysis)?, antibodies-online
Flow Cytometry, Wikipedia
Antibody, Wikipedia

Pingback: Approaches in Biomedical Research: Flow Cytometry, Part 2 – Advances in ME/CFS
Will you be researching the 2-5A synthatase pathway?
LikeLiked by 1 person
Hello,I read your blog named “Approaches in Biomedical Research: Flow Cytometry, Part 1 – Advances in ME/CFS” regularly.Your humoristic style is witty, keep doing what you’re doing! And you can look our website about free spells love.
LikeLike
I often visit your page and have noticed that you don’t update it
often. More frequent updates will give your website higher authority & rank in google.
I know that writing content takes a lot of time, but you can always help yourself with miftolo’s tools
which will shorten the time of creating an article to a few seconds.
LikeLike
Pingback: JAX ME/CFS CRC Scientific Progress – 2019 – Advances in ME/CFS
Pingback: An Update from the JAX ME/CFS Center – Advances in ME/CFS